- Joined
- Aug 1, 2003
- Messages
- 6,712
- Reaction score
- 887
South African doctor who first spotted the Covid omicron variant says symptoms seem 'mild' so far
By Holly Ellyatt | Nov 29 2021
Covid symptoms linked to the new omicron variant have been described as "extremely mild" by the South African doctor who first raised the alarm over the new strain.
Dr. Angelique Coetzee, chair of the South African Medical Association, told the BBC on Sunday that she started to see patients around Nov.18 presenting with "unusual symptoms" that differed slightly from those associated with the delta variant, which is the most virulent strain of the virus to date and globally dominant.
"It actually started with a male patient who's around the age of 33 ... and he said to me that he's just [been] extremely tired for the past few days and he's got these body aches and pains with a bit of a headache," she told the BBC.
The patient didn't have a sore throat, she said, but more of a "scratchy throat" but no cough or loss of taste or smell — symptoms that have been associated with previous strains of the coronavirus.
Coetzee said she tested the male patient for Covid, and he was positive, as was his family, and then said she saw more patients that day presenting with the same kinds of symptoms that differed from the delta variant.
This prompted her to raise the alarm with South Africa's vaccine advisory committee, of which she is a member.
Other patients Coetzee had seen so far with the omicron variant had also experienced what she described as "extremely mild" symptoms, and she added that her colleagues had noted similar cases.
"What we are seeing clinically in South Africa — and remember I'm at the epicenter of this where I'm practicing — is extremely mild, for us [these are] mild cases. We haven't admitted anyone, I've spoken to other colleagues of mine and they give the same picture."
Investigations ongoing
The WHO has said it will take weeks to understand how the variant may affect diagnostics, therapeutics and vaccines.
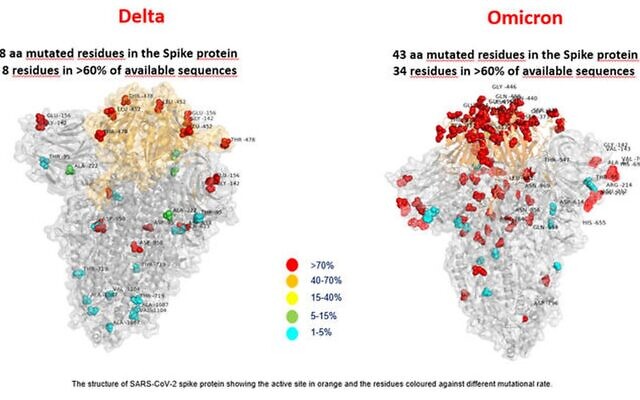
Coetzee's initial observations are only based on a very small number of cases and experts are worried about omicron's large number of mutations. Preliminary evidence suggests the strain has an increased risk of reinfection, according to the WHO.
Early data suggests that the variant is spreading in South Africa more rapidly than previous strains did and that the variant, known formally as B.1.1.529, could be starting to trigger a new wave of infections, according to analysis by the Financial Times.
It could take a while to fully understand what specific symptoms, if any, are attributable to the new omicron variant on a wider scale.
Covid symptoms have changed since the virus first emerged in China in late 2019. The "alpha" and "delta" variants, first discovered in the U.K. and India, respectively, were seen to cause different symptoms, for example, with the latter causing more headaches, sore throat, runny nose and fever.
The U.S. CDC has highlighted the variety of Covid symptoms that have been reported, noting "anyone can have mild to severe symptoms" that may appear two to 14 days after exposure to the virus.
On the list of symptoms from the CDC are fever or chills, a cough, fatigue, shortness of breath or difficulty breathing, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or a runny nose, nausea or vomiting and diarrhea.
Unnecessary panic?
A swath of countries has now temporarily banned travel from several southern African countries where the variant has been found, a move slammed as a "knee-jerk, draconian" reaction by South Africa's health minister on Friday.
Asked by the BBC's Andrew Marr whether countries like the U.S., U.K., Israel and EU were "panicking unnecessarily," Coetzee stressed that the omicron variant had already likely spread to those nations.
"I think you already have it there in your country without even knowing it so I would say at this stage, definitely. Two weeks on, maybe we will say something different," she added.
Margaret Harris, spokesperson for the WHO, told CNBC on Monday that "we have South Africa to thank" for raising the alarm over the new variant, which has already been found in the U.K., France, Israel, Belgium, the Netherlands, Germany, Italy, Australia, Canada and Hong Kong, but not yet in the U.S.
Harris said the organization didn't like to see travel restrictions but understood that countries needed to take precautions based on their own epidemiological situations and risk-based analysis of the current data.
The U.N. health agency said Monday that the delta variant is still responsible for most of the current infections globally and, as such, was still its biggest concern.
"Over 99% of cases around the world are due to the delta variant and more deaths are occurring in the unvaccinated," WHO Chief Scientist Dr. Soumya Swaminathan told CNBC's "Squawk Box Asia" on Monday.
"I think that's our priority while we wait to find out more about [the omicron] variant."
Whether fresh restrictions and lockdowns might have to be introduced to counteract this new variant remain to be seen, experts say.
"The big issue and the big uncertainty is how severe illness will be with this new variant. There are signs from South Africa that, maybe, the illness is less severe than with delta but we don't really know if that is the case, and if it is, why that's happening," Paul Hunter, professor of medicine at the Norwich School of Medicine at the University of East Anglia, told CNBC on Monday.
"Maybe that's because it's reinfecting people who have already got some degree of immunity," he added. "If it generally is causing mild disease and if, as I suspect, the booster campaign will go a long way to still reduce hospitalizations and deaths, hopefully we won't have to live under restrictions again."
https://www.cnbc.com/amp/2021/11/29/omicron-covid-variant-symptoms-heres-what-we-know-so-far.html
One can only hope, but we need more data
Yup she’s only referencing built African alphas in a land where the genetically weak succumb to death.
Overweight chubbies and grandma who’s on 20 different prescriptions to stay alive still at risk.
Shut her back down
Last edited: